PRESENTING AUTHOR: Dr Barry Hardy, Douglas Connect
AUTHORS: Barry Hardy and Markus Hegi (Douglas Connect), Glenn Myatt (Leadscope), Nina Jeliazkova (Ideaconsult), Micha Rautenburg (in silico toxicology), Pekka Kohonen and Roland Grafstrom (Karolinska Institute)
ABSTRACT
The SEURAT-1 (Safety Evaluation Ultimately Replacing Animal Testing-1) research cluster is comprised of seven EU FP7 Health projects and is co-financed by Cosmetics Europe. The SEURAT-1 strategy is to adopt a mode-of-action framework to describe repeated dose toxicity to derive predictions of in vivo toxicity responses.
ToxBank is the cross-cluster infrastructure project which provides a web-accessible shared repository of research data and protocols. Experiments generate dose response data over multiple timepoints using different omics platforms including transcriptomics, proteomics, metabolomics, and epigenetics over different cell lines and a common set of reference compounds (details available at wiki.toxbank.net).
Data is also generated from functional assays and bioreactors and supplemented with in silico approaches. This complex and heterogeneous data is consolidated and harmonized through the ToxBank data warehouse. The approach includes the use of the ISA-Tab standard to describe experimental metadata and OpenTox services supporting interoperable data integration and analysis.
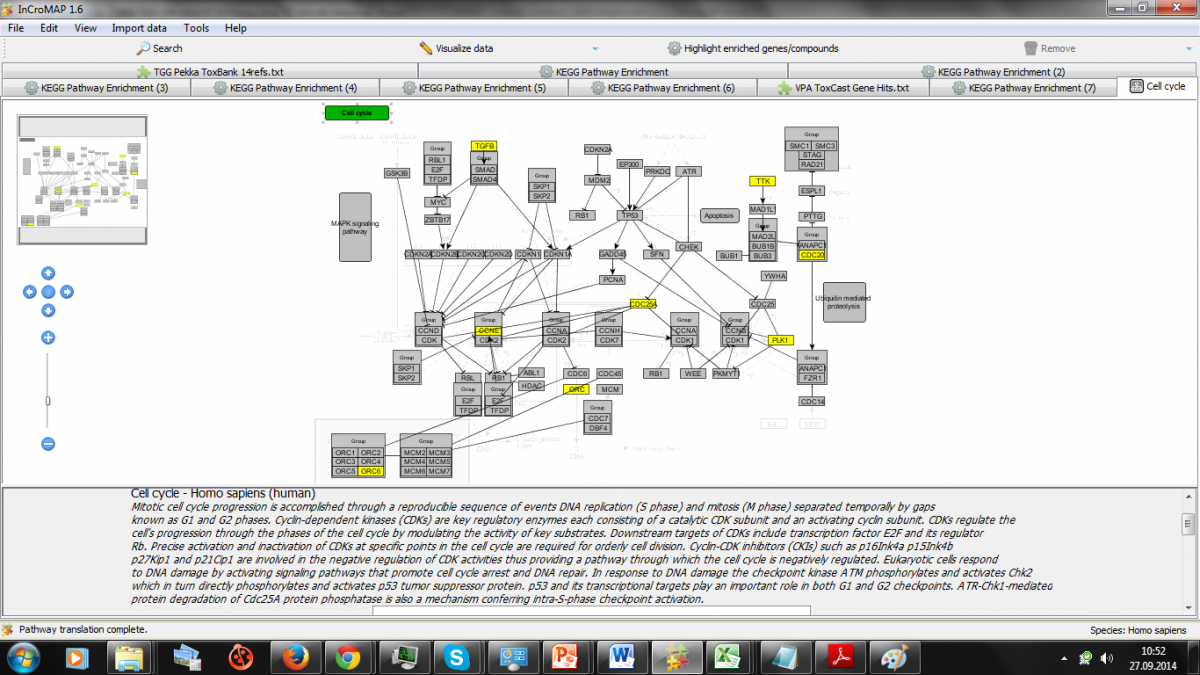
We describe for 14 reference compounds the meta-analysis of multiple types of time-dependent dose response omics and functional data combined with in vitro and in vivo background knowledge including consideration of modeling variations in biokinetic responses.
Open TG-GATEs human in vitro liver data of the reference compounds includes reactive compounds (e.g., acetaminophen, CCl4), mitochondrial disruptors (e.g., Rotenone), promiscuous binders (e.g., valproic acid, amiadarone), nuclear hormone receptor ligands (e.g., tamoxifen, WY14643), selective binders (e.g. fluoxetine) and cardiotoxins (e.g., Doxorubicin, Nifedipine).
Adverse events of interest that are represented include cytotoxicity, fibrosis, steatosis, cholestasis and phospholipidosis.
Overall we obtained 31,717 differential expression results with 14 compounds from the 45 comparisons, with Doxorubicin for example providing over 5000 results. We evaluate the use of ToxCast and PubChem data in the enrichment analysis, read across and interpretation of the evidence on reference compounds as mapped to biological pathways.
SPEAKER'S BIOGRAPHY
Dr Barry Hardy
 Dr. Barry Hardy is the Managing Director of Douglas Connect, Switzerland.
Dr. Barry Hardy is the Managing Director of Douglas Connect, Switzerland.
He has coordinated the OpenTox project in predictive toxicology and the ToxBank infrastructure development project. He currently leads the infrastructure development for the IMI EBiSC stem cell banking project and eNanoMapper supporting nanotechnology safety assessment.
He has led the development of research and best practice activities in drug design and toxicology through founding the eCheminfo Community of Practice, InnovationWell and Scientists Against Malaria project.
Currently he is developing Douglas Connect and its Collaboration Pools supporting new business area development and growth.
Dr. Hardy obtained his Ph.D. in 1990 from Syracuse University working in computational science. He was a National Research Fellow at the FDA Center for Biologics and Evaluation, a Hitchings-Elion Fellow at Oxford University and CEO of Virtual Environments International.
He was a pioneer in the early 1990s in the development of Web technology applied to virtual scientific communities and conferences. He has developed technology solutions for internet-based communications, tutor-supported e-learning, laboratory automation systems, and computational science and informatics.
In recent years he has been active in the field of knowledge management as applied to supporting innovation, communities of practice, and collaboration.